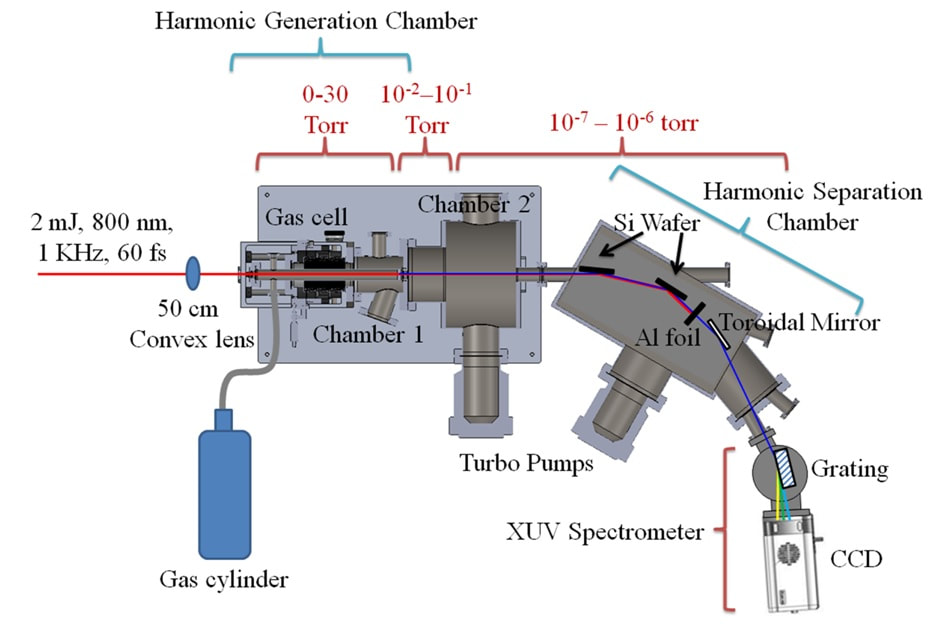
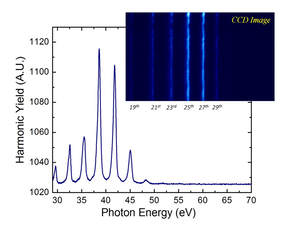
Experimental Facilities
Femtochemistry of Heterogeneous NanoCatalysis
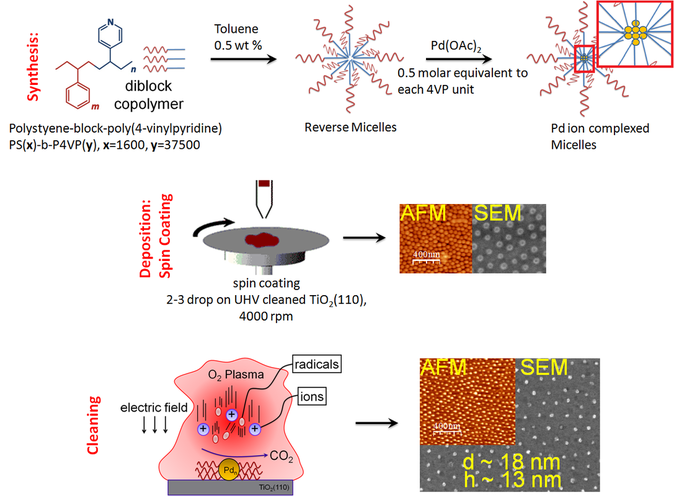
1. Preparation of Quasi-hexagonal 2D Arrays of sub-10 nm Particles: Model Catalyst Surfaces
Left: Synthesis Procedure (Micelle Nanolithography); Right: (a) Spin-Coater and (b) N2 Glove Box.
see Journal of Physical Chemistry C, 2018, 122, 26528-26542 for more details.
2. Study of Nanocatalysis using Surface Science Techniques
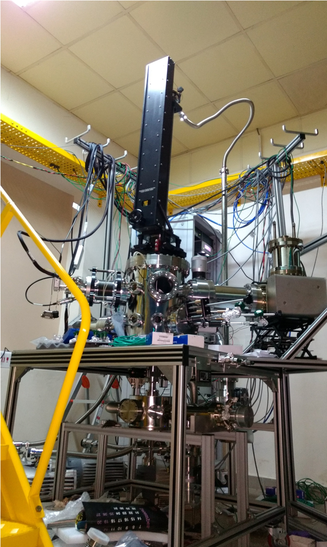
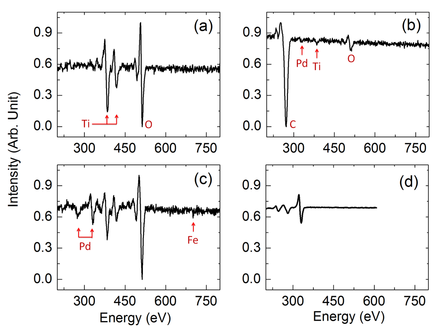
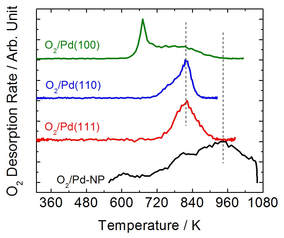
Left: Home-built surface analysis system; Right Top: Auger electron spectra of (a) single crystal TiO2(110), (b) TiO2(110)-supported loaded micelles, (c) clean TiO2(110)-supported Fe-Pd nanoparticles and (d) single crystal Pd(100). Right Bottom: Temperature programmed desorption of O2 from TiO2(110)-Supported Pd Nanoparticles and single crystal Pd(111), Pd(110) and Pd(100) surfaces.
|
Equipped with Quadrupole Mass Spectrometer, Cylindrical Mirror Electron Energy Analyzer, Sample Probe with Temperature Ramp from 100 K to 1100 K, Ion Gun, Gas Doser, Plasma Source, XYZ Manipulator, Turbo Molecular Pump, Ion Pump, and Laser Windows.
|
Nanoconfinement Effect: Atomic oxygen binding strength of Pd nanoparticles (~10 nm) is significantly enhanced with respect to the Pd lower Miller index surfaces due to nanoconfinement effect.
|
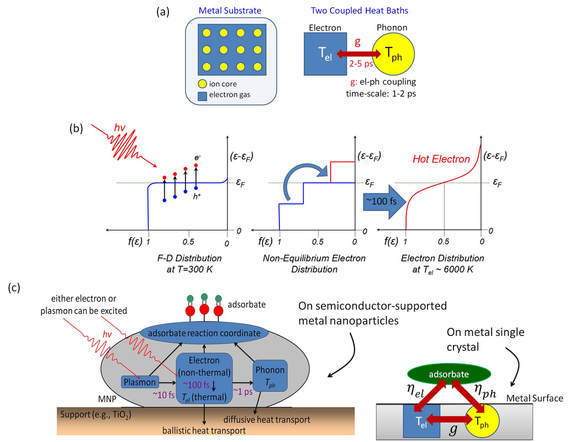
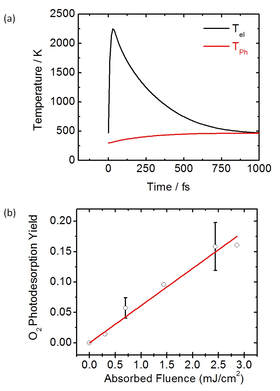
3. Ultrafast Surface Chemical Dynamics Coupling Femtosecond Spectroscopy and Surface Science
Left: (a)
see J. Phys. Chem. C, 2018, 122, 26039-26046 for more details.